|
 |
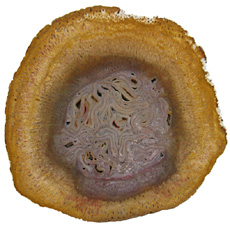
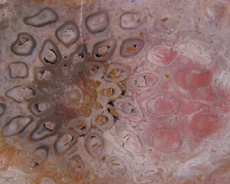
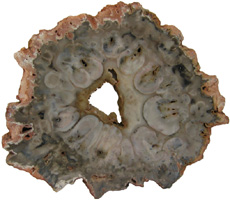
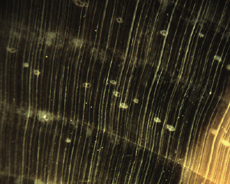
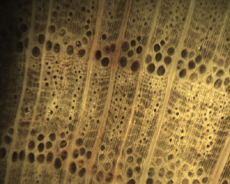
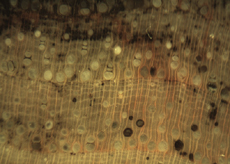
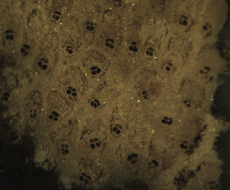
The Anatomy of Arborescent Plant Life Through Time  Tree Fern Guairea carnieri Paraguay, South America 20 cm x 12.5 cm |
Click anatomy for a revised, printable, version of our article. Revised January 2015
Introduction Collectors of petrified wood focus on permineralized plant material related to arborescent (tree-like) plant life. Fascination with fossil wood may be related to human reverence for living trees. Trees provide humans and other organisms with shelter and food. We plant trees near our homes and in our communities to enrich the environment. Trees define many biomes. Trees help moderate Earth’s atmosphere, sequestering carbon and releasing oxygen. Trees are one of the first plant categories a child learns. Asking a person to identify a plant as a tree may seem like “child’s play”; however, defining a tree can be difficult. The United States Forest Service defines a tree as a woody plant at least 13 feet (4 m) tall with a single trunk at least 3 inches (7.62 cm) in diameter at breast height (4.5 ft; 150 cm) (Petrides, 1993, p. 4). This definition fits well with many people’s concept of a tree being a large, columnar, woody, long-lived organism. However, many trees are not constructed from secondary growth (wood), such as palms and tree ferns. Some species, such as black willow, are multi-trunked. Size can also be problematic as an Engelmann spruce growing at tree line may be small compared to one growing at a lower elevation. Some species, such as the juniper, can grow as shrubs or trees. The Japanese art of bonsai demonstrates how environment can effect tree growth to extremes. We will adopt a more encompassing definition of the tree form. A tree is a perennial plant often having a single trunk supporting secondary branches with leaves or a crown of leaves. Evidence for the first fossil trees and forests occur in the Devonian. The oldest arborescent plant, Eospermatopteris, is related to ferns (Taylor, Taylor, and Krings, 2009, p. 479). Fossil forest composition changes through geologic time, reflecting variety in evolutionary strategies for constructing a tree form. It is helpful and informative to study the anatomy of various trunk designs. Plant Organs & Tissues Evolutionary adaptations for trunk structure can be recognized by the arrangement of tissues and organs. A quick survey of plant organs and tissues will enhance our discussion of the various evolutionary strategies for constructing a tree form. Plants are made of four types of organs: roots, stems, leaves, and reproductive structures. In turn, these organs are composed of three basic tissue systems: the ground tissue system, the vascular tissue system, and the dermal tissue system. Ground Tissues Ground tissues including parenchyma, collenchyma and sclerenchyma are involved in photosynthesis, storage, secretion, transport, and structure. Parenchyma tissue generates all other tissues. Living parenchyma cells are involved in photosynthesis, storage, secretion, regeneration and in the movement of water and food. Parenchyma cells are typically spherical to cube shaped. Collenchyma tissue provides structural support for young growing organs. Living collenchyma cells are elongated cylinders and help to make up the familiar string-like material in celery stalks and leaf petioles. Sclerenchyma tissue provides support for primary and secondary plant bodies. Sclerenchyma cells often have lignified secondary walls and lack protoplasm at maturity. Elongated slender sclerenchyma cells known as fibers make up well known fiberous material such as hemp, jute, and flax. Shorter sclerenchyma cells known as sclereids make up seed coats, the shells of nuts, and account for the gritty texture of pears. Vascular Tissues The vascular tissue system is represented by the water conducting tissue xylem and the food conducting tissue phloem. Xylem tissue is made primarily of parenchyma cells, fibers, and tracheary elements. Tracheary elements are represented by tracheids and vessel elements. Tracheids and vessel elements are enlongated cells that lack protoplasm at maturity and have secondary walls strengthened with lignin. Vessel elements are lager in diameter than tracheids and are an adaptation of flowering plants. Tracheary elements form an interconnected system of overlapping, leaky tubes that conduct water and minerals from the roots to the rest of the plant. Transpiration of water from leaves pulls columns of water enclosed within these stacked, tube-like cells up the plant. Phloem tissue is made primarily of sieve elements, parenchyma cells and fibers. Sieve elements are represented by sieve cells and sieve-tube members. Sieve cells and sieve-tube members are elongated cells that are living at maturity. Both cell types are closely associated with parenchyma cells. Sieve-tube members possess larger pores and are an adaptation of flowering plants. Sieve elements form an interconnected system of tubes that transport the food products of photosynthesis made within leaves throughout the plant. Dermal Tissues The dermal tissue system forms a protective outer covering including the epidermis and periderm. The epidermis forms the outer most layer of the primary plant body. During secondary growth the periderm replaces the epidermis. The periderm consists of protective dead cork tissue, the cork cambium, and phelloderm, a living parenchyma tissue. Meristems Tissues that make up plant organs are produced from clusters of actively dividing parenchyma cells called meristems. Cells produced from meristems differentiate into the specialized cells making up tissues. Apical meristems occur at the tips of roots and shoots. Apical meristems are responsible for primary growth or increase in length. Lateral meristems such as the vascular cambium and cork cambium (phellogen) produce secondary growth, increasing the girth of stems. Typically, the vascular cambium is bifacial, producing secondary xylem to the inside and secondary phloem to the outside. Secondary xylem makes up the wood of a stem, which will be explored in more detail when looking at modern conifers and angiosperms. The cork cambium produces phelloderm to the inside and phellem (cork) to the outside. Together, the phelloderm, cork cambium, and cork make up the periderm, a protective layer made by secondary growth (Raven, Evert, & Curtis, 1981, pp 417-429). The term bark refers to all tissues outside the vascular cambium. Steles Knowledge of how primary vascular tissues are organized within the central cylinder of stems and roots can aid in identification. The central cylinder of a sprout axis, which includes vascular tissue and associated ground tissue, is called a stele. Structurally, one can think of three basic ways in which the stele of stems and roots are organized based upon tissue found in the center of the axis. The center of a stele can be made of solid xylem, pith or vascular tissue embedded in ground tissue. A protostele has no pith; instead the center is a solid core of xylem. Most primitive plants and roots are protostelic. Three types of protostele are recognized by the shape of the xylem core in cross-section. In a haplostele, cylinder-shaped xylem is surrounded by a ring of phloem. Actinosteles have xylem with arm-like projections surrounded externally by phloem. Plectosteles possess plate-like regions of xylem surrounded by phloem. The plates look like they are separate but in fact are connected longitudinally. Siphonosteles have a pith surrounded by vascular tissue. Siphonosteles can have phloem on both sides of the xylem (amphiphloic) or only external to the xylem (ectophloic). Two variations of this structure can be recognized based upon how many interruptions in the vascular ring occur in any one cross-section due to the formation of leaf traces. These interruptions or breaks in the vascular ring are called leaf gaps. Solenosteles exhibit no more than one leaf gap in a cross-section. Dictyosteles exhibit multiple leaf gaps in cross-section. Solenosteles and dictyosteles are found in ferns. All seed plants have primary vascular tissue organized into bundles either surrouding a pith or scattered within ground tissue. Gymnosperms and dicotyledonous angiosperms have vascular strands arranged in a ring around the pith, called a eustele. A variation of the eustele, called the atactostele is found in angiosperm monocots in which the vascular bundles are scattered throughout the ground tissue. The roots of monocots are arranged as a eustele, while in gymnosperms and dicots, roots are protosteles. The eustele was once thought to be derived from the siphonostele, but multiple lines of evidence indicate it is derived from the protostele (Taylor, Taylor & Krings, 2009, p. 220). Trunk Structures All arborescent vegetation represent vascular plants with true roots, stems, and leaves. As we explore the anatomy of arborescent plant life we will discover that a variety of tissues and organs have been co-opted as strengthening elements. Several basic trunk structures can be recognized by the arrangement of strengthening elements: solid woody cylinders, fibrous cylinders composed of isolated, intertwined elements and reinforced tube-like cylinders with hollow or soft centers. Permineralized
plant material is often cut and polished in the cross-sectional
or transverse plane to reveal the
anatomy perpendicular to a trunk, stem, or root axis. Familiarity
with the anatomy may even allow one to identify the taxon
to which a specimen belongs; however, for many specimens
radial and tangential sections of the stem must also be studied.
In this article, we will focus only on stems in cross-section.
You can obtain some general information about major plant
groups by visiting the Science Olympiad section of our
website. The extinct clubmoss trees or arborescent lycopods dominated the canopy of Carboniferous forests and went extinct during the Permian. Paleozoic tree clubmosses could reach heights of 40 m and attain diameters of 2 m. The best cross-sections of lycopsid stems and trunks come from coal ball cellulose acetate peels. Evidence from coal ball fossils has allowed paleobotanists to reconstruct the growth and structure of these unusual trees.
As
branches grew at the top of the tree they once again became
protosteles with very small primary vascular
cylinders.
Thus, without secondary tissues these branches
lost their ability to grow larger. The tree literally
grew itself out (Taylor, Taylor, & Krings, 2009,
pp. 288
& 289). This determinate growth contrasts with
most woody plants, which have indeterminate growth. The horsetails (phylum Sphenophyta or Equisetophyta) range from the Devonian to recent times. Equisetum is the only extant (living) genus (Willis & McElvain, 2002, p. 104). Extant species representing this genus are all small herbaceous plants. Horsetails have jointed stems with vertical ribbing. All the branches, leaves and cones are borne on whorls (Kenrick & Davis, 2004, p. 89). Horsetails (sphenopsids) are closely related to ferns. Some taxonomists place the sphenopsids in the fern division Pteridophyta. Like the lycopsids, sphenopsids also have a rich evolutionary history that includes tree forms. Arboreal
horsetails contributed to the canopy of Paleozoic
forests. Calamites is the most well known
arboreal horsetail. The trunk of Calamites grew
in a telescoping fashion from one node to another
(Selmeier, 1996, p.
139). Like the lycopsids arboreal horsetails exhibited
determinate growth. The stems and roots of horsetail
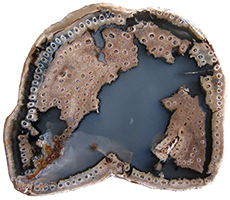
trees are siphonostelic. In cross-section the calamitean stem
consists of pith and or
a medullary cavity
surrounded by Ferns Ferns (division Pteridophyta or Filicophyta) range from the Devonian to recent times. Extant ferns are represented by over 12,000 species. While many ferns are smaller herbaceous plants, larger tree-like ferns still exist. A type of tree related to ferns was present in the oldest known forest. Middle
Devonian fossil trunks from Gilboa, New York provide
a Marattiales Ferns in the order Marattiales range from the Carboniferous to recent times. This was the first modern group of ferns to evolve a structure that we think of as a real tree fern. The Carboniferous arborescent ferns possessed a kind of buttressed or braced trunk. Psaronius was
the largest arborescent fern found in the coal measure
swamps (Willis & McElvain, 2002, p. 108). Psaronius was
up to 10 m tall with a 1 m wide trunk. These tree ferns
occupied the drier areas of the swamps and by the
end of the Carboniferous replaced arborescent lycopods
as the dominant trees in the swamp forests (Cleal
& Thomas, 2009, p. 111). Psaronius stems
start out as protosteles but as they mature the
central xylem strands become embedded in a
ground tissue making them a dictyostele. The
root mantle became narrower
towards the top of the tree, while the
Ferns representing the order Osmundales range from the Permian to recent times. Osmundales is a primitive group that may have important evolutionary ties to many modern fern families. Two families within this order had species that reached tree size. Guaireaceae
is an extinct family of ferns that ranges from
the Osmundaceae,
the Royal Fern family, makes its appearance in
the Permian. Sixteen living species are recognized
along with
nearly a hundred fossil forms (Miller, 1971). Osmundaceae
is the best-represented family of ferns in the
fossil record and
is known from foliage, stems, roots and reproductive
structures. The family diversified and was widespread
during the Mesozic
era, but decreased in numbers and geographic range
during the Tertiary (Tidwell, 2002, p. 135). The
ferns in this
family
have rhizomes that grow upright and produce closely
spaced fronds.
Not unlike Psaronius, the fossil osmundales
also Filicales Filicales (Polypodiales) is the largest order of ferns today with roughly 9,000 extant species. Filicales range from the Carboniferous to recent. Several families within this group are represented by excellent permineralized stems.
Permian
fossil forests in Brazil have proved to be a great Most
tree ferns employ a mantle of roots
and
petioles
around a vascular cylinder to form a
fibrous
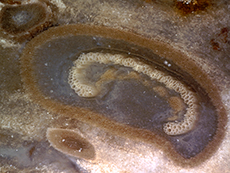
trunk. A very unique tree fern, Tempskya,
employed a very different strategy for constructing
a fibrous trunk. Tempskyaceae
are an extinct family of Mesozoic ferns, in the
order Filicales, represented by the single genus Tempskya (Tidwell,
2002, p. 153).Tempskya occurs
as the silicified false trunk of a Cretaceous aged
tree fern. Tempskya is referred to as
a false trunk Several stems initiated the growth of the tree fern and branched dichotomously in a uniform profuse manner throughout life, producing both the apical and lateral growth of the false trunk. Roots, emerging from the sides of stems, branched profusely filling in voids, tying the mass of stems together forming the false trunk. Roots, greatly outnumbering the stems provided the structural support for the false trunk. Roots growing down ruptured stems and petioles. Upper portions of stems continued to be nourished by emerging adventitious roots. Consequently, transverse sections near the base of the false trunk have few stems and many roots while transverse sections towards the apex of the false trunk have many stems embedded among the roots. Evidence suggests that Tempskya was a short to medium tree fern with diameter of up to 30 cm and heights reaching up to 3 meters. The tree bore small leaves on its crown and for a considerable distance downward from the apex (Tidwell, 1998, p. 190; Andrews, 1943, p. 136; Andrews & Kerns, 1947, p. 155). To learn more, read our article on Tempskya.
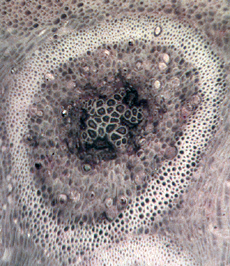
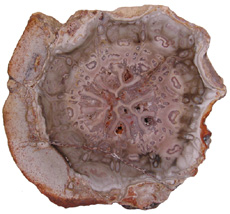
Cyathodendron has an irregular shaped solenostele with numerous medullary bundles. In cross-section one can see numerous vascular bundles embedded within the central pith. Vascular bundles within the pith area are called medullary bundles. Ribbon-like vascular tissue surrounds the pith. The ribbon-like vascular tissue and the medullary bundles contribute to the origination of leaf traces. Adventitious roots arise from the leaf traces. In cross-section petioles exhibit numerous vascular strands. The mantle making up the exterior of Cyathodendron is composed of leaf bases and multicellular epidermal hairs (Tidwell, 1998, pp. 192 & 193; Taylor, Taylor & Krings, 2009, p. 466). Although not an arborescent plant, the beautifully permineralized petioles of the giant fern Acrostichum are worthy of mention. Acrostichum is an extant genus of the leatherferns within the family Pteridaceae. Acrostichum is composed of erect rhizomes bearing very large fronds, up to 3.5 meters in living species. Today these ferns are often found in marsh environments. Evidence for this same type of environment is found for fossil forms as well (Tayolon, Taylor & Krings, 2009, p. 471).
Progymnosperms (Division Progymnospermophyta) range from the Devonian to the Carboniferous and are thought to represent the plant group from which all seed plants evolved. Progymnosperm trees reached heights of 8 meters and diameters up to 1.5 meters (Willis & McElwain, 2005, p. 110). Progymnosperms are believed to link ferns to gymnosperms. In 1960 the American Paleobotanist Charles Beck published a paper describing the connection between the foliage known as Archaeopteris and the wood Callixylon. Archaeopteris was believed to be a fern, while Callixylon was thought to represent a gymnosperm. The work of Beck demonstrated that the foliage known as Archaeopteris and wood known as Callixylon belonged to the same plant. Archaeopteris is a true missing link between fern-like plants and conifer-like plants (Kenrick and Davis, 2004, pp. 41-43). The trunk (Callixylon) of this tree was constructed of conifer-like wood, while the branches were adorned with fern-like fronds (Archaeopteris). The underside of the fronds had sac-like sori that contained spores for reproduction.Progymnosperms
are the earliest trees with modern wood anatomy
and growth habit (Taylor, Taylor, and Krings,
2009, p. 479). Progymnosperms stems are eusteles.
In cross-section the trunk of Archaeopteris (Callixylon)
was very much like modern conifers consisting
of a small central pith surrounded by secondary
xylem
and bark. The solid cylinder construction could
support profuse branching. Archaeopteris made
up a significant portion of the canopy of early
Devonian forests. In plants that produce seeds (gymnosperms and angiosperms) the two phases are bound into a single individual. Gymnosperms produce very small gametophytes within male and female cones or on specialized branches or leaves. The male gametophyte is the pollen, while the female gametophyte consists of eggs in the ovule. The sperm of gymnosperms do not swim through water to reach the ovules (Ginkgo biloba and cycads are the exception, see Taylor, Taylor, and Krings, 2009, p. 744). Wind carried pollen transports the sperm. When pollen lands near an ovule a pollen tube grows towards the ovule. Sperm travel down the pollen tube to fertilize the eggs in the ovule. Seeds provide many advantages for both plants and animmals. Seeds encase the developing plant embryos, providing them with nutrients and protection from the surrounding environment. Seeds allow embryos to remain dormant during unfavorable conditions. Pollen frees gymnosperms from the need to have water for fertilization. Seeds allow gymnosperms to delay germination until favorable conditions exist. These reproductive strategies gave gymnosperms a distinct advantage over the spore producing plants in dry environments. The seeds also became a valuable source of food for animals. Gymnosperms Seed
Ferns
Schilderia Hermanophyton Hermanophyton represents the genus of an extinct gymnosperm stem found in Jurassic aged deposits of Colorado and Utah. The genus is also represented by one Late Cretaceous aged specimen from the Aken Formation collected in the Bingeberg-Flöeg sandpit in Hauset, Belgium (Knoll, 2010, pp. 181-185).
Hermanophyton was most likely a small to medium narrow stemmed tree reaching heights of 18 meters and crowned with small leaves which dropped off as the plant grew leaving behind numerous, small, persistent leaf bases (Tidwell & Ash, 1990, pp. 87 & 88). Fossil stems of Hermanophyton maintain a fairly consistent diameter over substantial lengths. The stem acted as a solid cylinder; however, there is little evidence of branching. No leaves or reproductive structures have been associated with Hermanophyton so its affinity with other gymnosperms is uncertain. Thus, it is an incertae sedis gymnosperm (Tidwell & Ash, 1990). To learn more, read our article on Hermanophyton. Cycads & Cycadeoids Cycadopytes (division Cycadophyta) made up a significant portion of the Mesophytic flora. The Mesozoic is sometimes referred to as the "age of cycads". Cycadophytes are gymnosperms that have a superficial resemblance to the flowering palms. Cydadophyte trunks range from short and squat to tall and columnar or tree-like. Stems are covered with a protective layer of persistent leaf bases. Leaves can be scale-like or in the form of fern-like fronds. One living and one extinct order are recognized within this division. True
cycads,
both living and extinct belong to the order
Cycadales. Cycads range from the Permian
to recent times. The
extinct order Cycadeoidales or Bennettitales
range from the Triassic to the late Cretaceous.
In the U.S. this order is referred to as Cycadeoidales,
while in Europe it is known as Bennettitales.
In cross-section both cycad and cycadeoid stems
are eusteles Charmorgia and Lyssoxylon are two important fossils found in the Chinle formation of Arizona that belong to Cycadales (Tidwell, 1998, pp 196-197). Many Mesozoic species had slender branching forms. Extant short, squat cycad forms did not appear until the Tertiary (Willis & McElwain, 2002, p. 135). One group of cycadeoids had a short barrel-like trunk crowned with large fronds. A second group was more shrub or tree-like. Perhaps the most well known cycadeoid genus is Williamsonia. Williamsonia resembled a shrub or tree and grew to a height of 3 meters. Williamsonia reached its greatest diversity during the Jurassic. The cones of cycadeoids are remenescent of flowers. Some paleotologists have suggested that the cycadeoids may have a close evolutionary relationship with angiosperms (flowering plants), although current evidence makes this tie unlikely. Both Cycadales and Cycadeoidales most likely evolved from medullosan seed ferns (Willis & McElwain, 2002, pp. 136-137). Ginkgo The Maidenhair tree or Ginkgo biloba is the only living species representing the division Ginkgophyta. Representatives of the order Ginkgoales date back to the Permian, but the genus Ginkgo makes its first appearance in the Jurassic. In fact, Ginkgophytes reached their greatest diversity during the Jurassic. Fossil evidence indicates that at least 16 genera of ginkgophytes made up a significant part of the Mesozoic vegetation (Willis & McElwain, 2002, p. 139). Ginkgos declined in the Paleogene and Neogene, becoming nearly extinct (Tidwell, 1998, p. 102). Ginkgo
trees have a constellation of characteristics,
making their origin difficult to discern.
The Ginkgo tree grows to 30 m. The fan
shaped leaves of Ginkgo remind one of
a deciduous flowering plant. The reproductive
structures of the Ginkgo are more like
that of a cycad. The roots and stems
of Ginkgo are conifer-like.
In cross-section the Ginkgo tree
is a solid Pinophytes, more commonly known as conifers, range from the Carboniferous to recent times. Cordaitales and Voltziales are well-known extinct orders of the division Pinophyta. The order Pinales has both extinct and extant genera. Pinales includes such familiar plants as pine, spruce, Douglas-firs, firs, cypresses, cedars, junipers, larches, sequoias, and yews . Two primitive conifer orders have important evolutionary ties to modern day conifers in the order Pinales. Cordaites (order Cordaitales) are conifer like plants that range from the Pennsylvanian to the Permian. Cordaites grew as shrubs and trees. Cordaite trees possessed a slender trunk adorned with a branching crown of leaves. Cordaite stems were eusteles that possessed a rather large pith. As the tree grew the pith broke down to create a hollow area. Artisia is the pith cast of Cordaites. The stem was composed of secondary wood surrounded by bark. In a broad sense the mature trunk was a variation upon the tube-like structure. Cordaites had long leathery straplike leaves with many parallel veins The leaves were spirally arranged around the stem. Voltziales (order Voltziales), commonly known as walchias, range from the Carboniferous to the Triassic. Voltziales were large trees that possessed needle-like leaves. The trunk was a solid woody cylinder with pith, secondary xylem and bark. Voltziales are traditionally viewed as an evolutionary transition between Cordaites and modern families of Conifers (order Pinales) (Bhatnagar & Moitra, 1996, p. 167; Taylor, Taylor, & Krings, 2009, p. 828). Pinales originated from members of Voltziales, which in turn have their origins within the Cordaitales (Willis & McElwain, 2002, p. 150). The fossil record of modern Pinales families dates back to the Triassic (Taylor, Taylor, & Krings, 2009, p. 870). Several conifers, representing the family Araucariaceae, from the Triassic and Jurassic are sought by collectors. If you acquire a conifer from a Mesozoic wood deposit that is composed of moderate sized tracheids, uniseriate rays with no resin canals or wood parenchyma it is a good bet that it will have been identified as Araucarioxylon. Perhaps the most well known fossil wood specimens assigned to this genus are the permineralized trunks of the Petrified Forest National monument in Arizona. If a specimen with the same characteristics of Araucarioxylon is found in older, Paleozoic deposits it will be referred to as Dadoxylon. It has become increasingly clear that the wood ascribed to Dadoxylon and Araucarioxylon represent a wide array of Paleozoic and Mesozoic plants (Stewart & Rothwell, 1993, p. 416; Savidge, 2007, p. 324). Some have argued that the pith structure, as seen in cross-section, can be used to distinguish between Dadoxylon and Araucarioxylon, making the age of the material irrelevant. The pith of Dadoxylon is variable in size but large, while the pith of Araucarioxylon is small and visually non discernable (Wright, 2002, pp. 129). A precise identification of these early conifers would require transverse, radial, and tangent sections.
Today members of the order Pinales can be found in just about any environment in the world. In some biomes, such as the Taiga, conifers are the dominant plant. Modern day conifers grow as shrubs or trees. Some arborescent conifers exhibit a pyramidal growth form. Leaves are usually needle-like, but can also show a broad flat shape. Conifers bear seeds in woody cones, except for junipers and yews, which have a berry-like structure. In
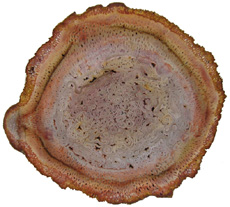
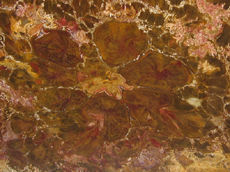
modern day New reproductive strategies helped angiosperms become a great success and diversify into the forms we know today. In angiosperms male and female structures develop within flowers. The pistil is the central, female organ of the flower and typically consists of an ovary with ovules, a style and stigma. The stamen is the male part of a flower and typically consists of a filament or stalk topped with pollen producing anthers. When pollen comes into contact with a flower's stigma the growth of a pollen tube is activated. Each pollen grain carries two sperm. One sperm fertilizes an egg in the ovule; the other sperm unites with two haploid cells in the same ovule. This process is known as double fertilization and is an important adaptation found in angiosperms. The fertilized egg will undergo cell division to become a zygote and then an embryo. The second fertilization results not in offspring, but rather the development of endosperm, which acts as a nutrient for the embryo. Cells in the endosperm have three sets of chromosomes (triploid). Endosperm not only serves as an important food source for the embryos of flowering plants it also is important to animals. Humans depend upon the endosperm of rice, wheat, and corn. A seed is formed when the endosperm and the embryo become enveloped in a part of the ovule that hardens into the seed coat. The ovary or other parts of the flower in angiosperms develop into a fleshy fruit surrounding the seeds. Many organisms such as birds, bats, and insects have coevolved to help pollinate angiosperms. The fleshy fruits of angiosperms are an adaptation for seed dispersal. Many animals use the fruit as a food source, which results in the dispersal of seeds encapsulated within a natural fertilizer! In addition to new reproductive strategies flowering plants also exhibit adaptations to their vascular tissues. Sieve-tube members in the phloem and vessel elements in the xylem are found in most flowering plants. In fact, the presence of vessels in fossil wood is usually a good indication that it is an angiosperm. However, one must keep in mind there are vesseless dicot families, such as Trochodendraceae and gymnosperms that possess vessels in the order Gnetales. Still, vessels are vital in fossil wood identification. Angiosperms Dicots Woody
dicots possess eustele stems, a
central pith surrounded by secondary wood and bark
(see picture above). The woody stems of arborescent
dicots
are strong and resistant to buckling. Vascular
cambium produces secondary xylem to the inside and
secondary phloem
to the
outside. Most
angiosperms have cell types that are distinctly different
in size making up their xylem tissue (wood). Vessel
elements are larger
diameter water conducting cells.
Monocots
In
|
Click on the word anatomy for a revised, printable version of our article. |
Bibliography |
Andrews, H.N. (1943). Notes on the Genus Tempskya. American Midland Naturalist, Vol. 29, No. 1 pp. 133-136. Andrews, H.N & Kern, E.M. (1947). The Idaho Tempskyas and Associated Fossil Plants. Annals of the Missouri Botanical Garden, 34, pp. 119-186. Arens,
N.C. Lab V Lycophytes, UCMP Berkley: Arnold, C.A. (1940). Lepidodendron johnsonii, sp. nov., from the Lower Pennsylvanian of Central Colorado. Contributions from the Museum of Paleontology, University of Michigan vol 6, no 2, pp. 21-52. Arnold, C. A. (1945). Silicified Plant Remains from the Mesozoic and Tertiary of Western North America I. Ferns. [Reprinted from Papers of the Michigan Academy of Sciences, Arts, and Letters, Vol. XXX, 1944] Arnold C. A. & Daugherty L.H. (1963). The Fern Genus Acrostichum in the Eocene Clarno Formation of Oregon. Contributions from the Museum of Paleontology, The University of Michigan vol. 28, no 13, pp. 205-227. Bhatnagar S.P. & Moitra, A. (1996). Gymnosperms. India: New Age Publishers. Bland, R.G. and Jaques, H.E. (1978). How to Know the Insects [3rd Ed.] Dubuque, IA: WCB McGraw-Hill Brown, R.W. (1936). Field identification of the fossil ferns called Tempskya. Journal of the Washington Academy of Sciences Vol. 2, No 2, pp. 45-52. Cleal C.J. & Thomas, B.A. (2009). Introduction to Plant Fossils. United Kingdom: Cambridge University Press. Dernbach, U., Noll, R., & Robler, R. (2002). News From Araguaina, Brazil. In Dernbach, U. & Tidwell, W.D. Secrets of Petrified Plants: Fascination from Millions of Years (pp. 78-87). Germany: D’ORO Publishers. Elias, T.S. (1980). The Complete Trees of North America. New York: Van Nostrand Reinhold Company. Esau, K. (1977). Anatomy of Seed Plants [2nd Ed.]. New York: John Wiley & Sons. Hoadley,
B.R. (1990). Identifying Wood: Accurate Results with
Simple Tools. Newton, Connecticut: Taunton Books & Videos. Knoll,
H. (2010a). Het Late Krijt van Aken en omgeving: Deel
1-Verkiezeld hout, dennenappels en Meer. Natuurhistorisch
Maandblad 99 (8): 181-185. Mauseth,
J.D. (2014). Botany: An Introduction to Plant Biology [5th
Ed.]. Burlington,
MA: Jones & Bartlett Learning. Petrides, G.A. (1993). Peterson First Guide to Trees. New
York: Houghton Mifflin Company. Robler, R., & Galtier, J. (2002). First Grammatopteris tree ferns from the Southern Hemisphere-new insights in the evolution of Osmundaceae from the Permian of Brazil. Review of Palaeobotany and Palynology, vol 121, pp. 205-230. Savidge, R.A. (2007). Wood anatomy of Late Triassic trees in Petrified Forest National Park, Arizona, USA, in relation to Araucarioxylon arizonicum Knowlton, 1889. Bulletin of Geosciences vol. 82, 4, pp. 301-328. Selmeier, A. (1996). Identification of Petrified Wood Made Easy. In Dernbach, U. Petrified Forest: The World's 31 Most Beautiful Petrified Forests (pp. 136-147). Germany: D’ORO Publishers. Shogren,
E. (2008). Outlook Bleak for Joshua Trees.
NPR Stein, C.L. (1982). Silica Recrystallization in Petrified Wood. Journal of Sedimentary Petrology, vol 52, no 4. pp. 1277-1282. Stein, W.E., Mannonlini, F, VanAller Hernick, L., Landing, E. & Berry, C.M. (2007). Giant cladoxylpsid trees resolve the enigma of the Earth's earliest forest stumps at Gilboa. Nature, vol 446: pp. 904-907. Stewart W.N. and Rothwell G.W. (1993). Paleobotany and the Evolution of Plants [2nd edition]. Cambridge University Press: Cambridge. Taylor,
E.L. (1991). The Occurrence of a Rhexoxylon-like
stem in Antarctica. Courier Forschungsinstitut Senckenberg 147:
183-189 [15]. Tidwell, W.D. and Ash, S.R. 1990. On the Upper Jurassic Stem Hermanophyton and its Species from Colorado and Utah, USA. Palaeontographica 218, 77-92. Tidwell, W.D. and Parker, L.R. (1990). Protoyucca shadishii gen. et. sp. nov., An Arborescent Monocotyledon with Secondary Growth from the Middle Miocene of Northwestern Nevada, USA. Review of Palaebotany and Palynology. 62, pp. 79-95. Tidwell,
W.D. 1998. Common Fossil Plants of Western North
America. [2nd Edition]. Smithsonian Institution
Press: Washington, pgs 214-215. Tidwell,
W.D. (2002). The Guaireaceae-an Extinct Fern Family. In
Dernbach, U. & Tidwell, W.D. Secrets of Petrified
Plants: Fascination from Millions of Years (pp.
148-151). Germany: D’ORO Publishers. Walters,
D.R. & Keil, D.J. (1996). Vascular Plant
Taxonomy [4th Ed.]. Dubuque, IA: Kendell/Hunt. Wright, W. (2002). The Triassic Chinle Formation, USA, and its Fossil Woods. In Dernbach, U. & Tidwell, W.D. Secrets of Petrified Plants: Fascination from Millions of Years (pp. 121-133). Germany: D’ORO Publishers.
|