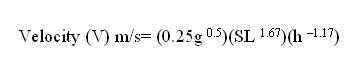|
 |
 Trilobite Tracks Kope Formation Ordovician Cininnati, OH |
||
Fossils do not always represent a part of the organism. Trace fossils record the activities of organisms. Tracks, burrows, eggshells, nests, tooth marks, gastroliths (gizzard stones), and coprolites (fossil feces) are examples of trace fossils or ichnofossils. Trace fossils represent activities that occurred while the animal was alive. Thus, trace fossils can provide clues to diet and behavior. Ichnology (ichn "trace or track, -ology "the science of") is the study of trace fossils. Trace fossils represent multiple modes of preservation but are considered here as a category for convenience. Tracks Tracks represent animals going about their day-to-day activities and may provide insight into the dynamic behavior of extinct organisms. Tracks are formed in situ, that is they are found in the place were the organism made them. The rocks containing tracks provide clues to the environment in which the imprints were made. Footprints making up a track can reveal the pace (steps), stride (the distance between consecutive steps made by the same foot), and trackway width or straddle. Steps and stride can reveal anatomical features, such as, number of toes or whether the organism was a biped or quadruped. Straddle can be used to measure the extent to which the animal sprawls or walks erect (Lockley & Meyer, 2000, pp3-4). Pace angulation (angle between step line segments) helps to determine the body width of an animal (Prothero, 1998, p. 413). Mathematical relationships between stride length and hip height (measured by footprint length) of some vertebrates can help us to establish relative velocity.
Trackways
can provide clues to the social nature of the
animal, was it gregarious (social), and did the
animal travel in herds? Trace fossils can be combined
to provide
multiple lines of evidence. Dinosaur nesting sites
and trackways
support the idea that some herbivorous dinosaurs
were gregarious. This same evidence may also point
to migrating
behavior. Tracks
of multiple organisms (footprint assemblage or
ichnocoenosis)
combined with an analysis of rock formation can
help to build
an ecological
picture
of
ancient environments. Trackways at Davenport Ranch
in Texas record a herd of 23 sauropods apparently being
tracked by 5 theropod dinosaurs (Prothero, 1998, p.
414) In
very rare instances the tracks are found with the maker.
A specimen of Kouphichnium walchi (a horseshoe
crab), found in the Solnhofen strata, is
preserved at the end of its track (Boucot, 1990, p.
314). Bivalves with escape trails have been found
in siderite nodules from Mazon Creek (Nudds & Selden,
2008, p. 123). The
Reverend William Buckland (1784-1856), an English geologist/paleontologist,
was the first scientist to recognize the true nature
of fossilized feces. Buckland coined the term coprolite
or
"dung-stone"
to describe these trace fossils. The oldest known vertebrate
coprolites come from Silurian deposits and represent
fish feces
(Eschberger, 2000, Coprolite Article). Coprolites attributed
to arthropods are known from the Ordovician (Taylor,
Taylor & Krings, 2009, p. 1007). Coprolite research is carried out primarily with thin sections. Herbivorous dinosaur coprolites studied by Chin have established a relationship between plant eating dinosaurs and tunneling insects related to dung beetles (Eschberger, 2000, Coprolite Article). Dr. Chin has also established that T-rex pulverized its victims from studying microscopic bone fragments found in a coprolite. Indian and Swedish scientists have found grass phytoliths in dinosaur coprolites. This helps to establish that dinosaurs and grasses coexisted (Williams, 2008, p. 51). Coprolites of arthropods recovered from rock material, such as the Rhynie chert, are composed of spores as well as plant and fungal remains (Taylor, Taylor & Krings, 2009, p. 1007). These Devonian-aged arthropods were probably detritus feeders. Coprolites provide important insight into diet, physiology as well as the geologic and geographic distribution of plants and animals. Insect Ichnofossils Insect ichnofossils (trace fossils) can be helpful in determining what types of insects were present at a particular time and provide information about the nature and persistence of past plant-insect associations. Evidence for herbivory in insects appears in the Carboniferous. Like vertebrates, the first insects were carnivores and detritivores. Herbivory requires hosting cellulose-digesting bacteria through a symbiotic relationship within the gut. The oldest examples of marginal and surface feeding are on Carboniferous seed fern leaves of Neuropteris and Glosspteris (Grimaldi & Engel, 2005, p. 52). It is estimated that only 4% of the leaves in Carboniferous deposits exhibit damage from feeding. Herbivores do not make a significant impact on plant life until the Permian (Kenrick & Davis, 2004, pp. 166-167). Galls are excessive growths on stems, leaves, cones, and flowers caused by insect feeding or egg laying. The earliest fossil galls are found on the petioles of Psaronius tree ferns of the Late Carboniferous. Insect gall fossil diversity and abundance takes off with the advent of flowering plant evolution in the Cretaceous (Grimaldi & Engel, 2005, p. 53). Insects produce tunnels in wood known as borings or galleries. Some insects eat the cambial layer while others eat fungus that grows within the galleries, still others eat the wood itself. The oldest borings and galleries in wood, attributed to mites, are known from the Carboniferous. The first definitive beetle borings are from the Triassic. There are some borings in permineralized Triassic-aged wood from Arizona that are attributed to termites or bees; however, they may be beetle borings (Grimaldi & Engel, 2005, p. 54 & 55). Leaf mines are meandering tunnels produced by the feeding larvae of some beetle, fly, and sawfly species. The first definitive leaf mines first appear in the leaves of Triassic conifers and pteridosperms. Interestingly, the abundance and diversity of fossil leaf mines coincides with the radiation of flowering plants (Angiosperms) during the Cretaceous. Leaf mines have been used to establish the persistence of insect and plant associations. For example, the larvae of certain moth families have been eating the leaves of Quercus (oak) and Populus (poplars) for 20 million years and hispine beetles have been eating the leaves of Heliconia for 70 million years (Grimaldi & Engel, 2005, p. 52). Caddisfly larvae live in lakes, ponds, and rivers. Many build distinctive protective cases from bits of sand, shells and vegetation. Fossil caddisfly cases can often be identified to the family or even genus level. The oldest larval caddisfly cases (Trichoptera) are found in the Jurassic (Grimaldi & Engel, 2005, p. 51). Celliforma is a fossil bee nest (in the form of subterranean excavations) that is first found in Late Cretaceous deposits. Celliforma is found from the Cretaceous to the Pliocene (Grimaldi & Engel, 2005, p. 51). Termite borings appear in the Cretaceous and represent the oldest undisputed fossil nest for social insects (Grimaldi & Engel, 2005, p. 54). Coprinisphaera is the fossil burrow of a scarabaerine dung beetle, which makes its first appearance during the Paleocene. Coprinisphaera lived from the Paleocene to the Pleistocene and had a wide geographic range being found in South America, Antarctica, Africa and Asia. Coprinisphaera coincide with the evolution of the first ecosystems to have abundant mammalian herbivores. Evidence for the first scarab tunnels are found in the coprolites of herbivorous dinosaurs from the Late Cretaceous of Montana (Grimaldi & Engel, 2005, p. 50). Marine Trace Fossils Marine Trace fossils are often classified into behavioral categories. Does the trace fossil represent resting, dwelling, crawling, grazing or some other type of feeding behavior (see Prothero, 1998, p 406 for more details)? Perhaps the most practical way to classify trace fossils is by their associations with a particular sedimentary environment or ichnofacies. In marine environments different ichnofacies are associated with different water depths, physical energies (wave & current conditions), or even type of substrate. Ichnofacies have become a standard tool for sedimentary geologists as well as paleontologists (Prothero, 1998, p. 406). Evolutionary Trends Trace fossils can also help to establish evolutionary trends. Deep burrows in marine sediments first appear in the fossil record during the late Precambrian and indicate the presence of soft-bodied coelomates (Prothero, 1998, p. 227). The earliest evidence for herbivory in insects appears in the Carboniferous. Specimens of Neuropteris and Glossopteris seed fern leaves have been found that show signs of marginal and surface feeding. Definitive leaf mines, which are produced only by insects with complete metamorphosis, first appear in the Triassic. The first undisputed bee nests and termite borings appear in Cretaceous deposits adding evidence to body fossils that social insects had evolved by this time (Grimaldi & Engel, 2005, pp 50-55). Conclusion Ichnofossils are important tools that help geologists interpret sedimentary environments, paleobathymetry, and provide clues to the diagenetic history of some sedimentary rocks. For the paleontologist and fossil collector ichnofossils represent fossilized behavior and provide important clues to paleoecology and paleoenvironments. |
||
 Pseudocoprolite or Cololite? Wilkes Formation Miocene S.W. Washington State 5.5 cm in length |
 Pseudocoprolite or Cololite? Wilkes Formation Miocene S.W. Washington State 11 cm in length |
|
 Bird Tracks Green River Formation Eocene Wasatch County, Utah Plate 15 cm x 15 cm |
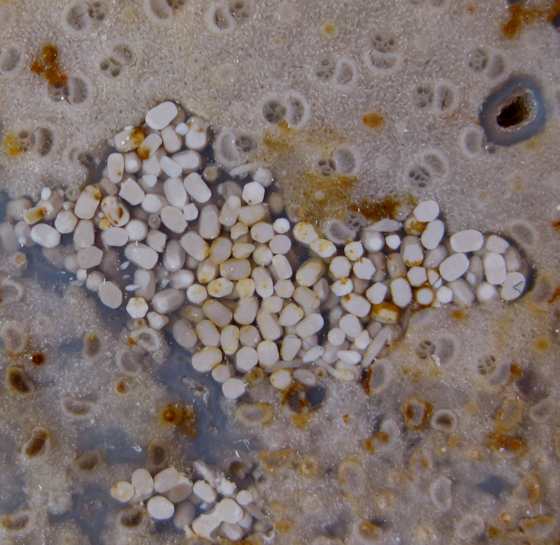
 Insect Galleries (White Ovals) Blue Forest Wood Green River Formation Eocene Blue Forest, Wyoming |
|
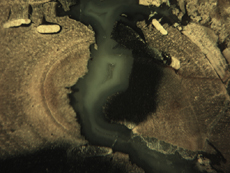
 Insect Galleries ("Sausage" Shapes by Central Pith) Green River Formation Eocene Blue Forest, Wyoming
|
 Teredolites ichnofacies (Teredo bivalve borings in Araucaria-like wood) Windalia Radiolarite Formation Middle Cretaceous Western Australia |
|
 Crocodile Coprolite Green River Formation Eocene Kemmerer, Wyoming 5 cm wide x 20 cm long A similar specimen is described by Grande (1984, p. 199). | ||
 Poplar Leaf with Insect Damage Populus wilmattae Green River Formation Eocene Bonanza, Utah |
||
 Ophiomorpha Burrow of a shrimp-like organism Larimer County, Colorado 1 cm wide x 7 cm long |
 Ophiomorpha cast Burrow of a shrimp-like organism Fox Hills Formation Cretaceous Weld County, Colorado 1.5 cm wide x 2 cm long |
|
|
||
Bibliography |
||
| Borror,
D.J. (1988). Dictionary of Word Roots and Combining Forms.
California: Mayfield Publishing Company. Boucot, A.J. (1990). Evolutionary Paleobiology of Behavior and Coevolution. New York: Elsevier Eschberger, B. (2000). Coprolites. Suite 101.com Garcia, F.A. & Miller, D.S. (1998). Discovering Fossils: How to Find and Identify Remains of the Prehistoric Past. Pennsylvania: Stackpole Books. Grande,
L. (1984). Paleontology of the Green River Formation,
with a Review of the Fish Fauna [2nd edition]. The
Geological Survey of Wyoming, Bulliten 63. Seilacher, A., Marshall, C., Skinner, H.C.W., Tsuihiji, T. (2001). A fresh look at sideritic "coprolites". Paleobiology, vol 27 No. 1: 7-13. Taylor,
T.N., Taylor E.L. & Krings, M. (2009). Paleobotany:
The Biology and Evolution of Fossil Plants [2nd
Ed]. New York: Academic Press. Williams, D. B., (2008) Its a Dirty Job, But Someone's Gotta Do It: Fossilized feces reveal significant details about ancient life. Earth, Sept. |
||