
 |
 |
 Hermanophyton taylorii Morrison Formation, Brushy Basin Member Upper Jurassic East McElmo Creek, Cortez, Colorado |
|
Introduction Preserved in the Morrison formation of Colorado and Utah are Jurassic aged conifers, cycads, and ferns. In the Henry Mountains of Utah collectors find conifers and cycads with great cell detail and fantastic coloration. The Yellow Cat area in Utah produces conifer casts with carnelian agate. Perhaps the most interesting gymnosperm from the Morrison deposits of Colorado and Utah is the gymnosperm Hermanophyton (Daniels, 1998, pp 100-119, 142, 153 & 154; Daniels & Dayvault, 2006, p. 103). Hermanophyton represents
the genus of an extinct plant stem found in Jurassic aged
deposits of Colorado and Utah. The genus is also represented
by one Late Cretaceous aged specimen from the Aken Formation
collected in the Bingeberg-Flöeg sandpit in Hauset,
Belgium (Knoll, 2010, pp. 181-185). It is the striking
anatomy of this stem in cross-section that first attracted
collectors to this rare fossil. The xylem
or water conducting tissue is arranged into large wedge-shaped
segments, see Figure 1. |
|
 Figure 1 |
|
Anatomy The stems of Hermanophyton range from 3 to 22.5 cm in diameter and up to 18 m in length. In transverse section the xylem cylinder is found to be composed of 9 to 15 wedge-shaped segments surrounding a central pith. The wedge shaped-segments are composed of primary and secondary xylem, the water conducting tissue of the plant. Primary rays radiating from the pith separate the wedge-shaped xylem segments. The primary rays merge with an outer two-layered cortex tissue, which in turn is surrounded by a ramentum see Figure 2. Hermanophyton stems maintain a fairly consistent diameter and are typically un-branched; only one branched specimen is known (Tidwell, 2002, p. 199). |
|
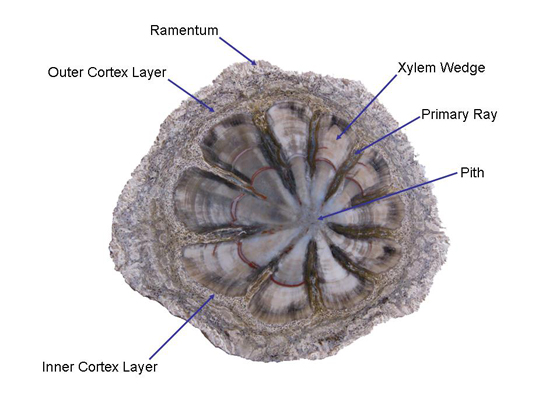 Figure 2 |
|
The pith, primary rays, and cortex are composed of parenchyma cells. The primary rays are continuous with the pith and cortex. Leaf traces originating from primary xylem travel down the primary rays. The vascular cambium is located around the outer margins of the xylem wedges. When alive the plant’s vascular cambium added secondary xylem to the growing woody wedge and a thin layer of phloem (food conducting tissue) to the outside. The inner cortex is made of densely packed cells and forms a thin layer around the xylem wedges. Parenchyma cells in the outer cortex are the same as that found in the central pith. The outer cortex contains many leaf traces or cortical bundles, many of which fuse to form the vascular tissue of the leaf bases found on the outer surface. The ramentum or outer covering consists of club-shaped structures referred to as capitate appendages composed of parenchyma cells. Hair-like fibers and leaf traces can also be found in the ramentum, see Figure 3 (Tidwell & Ash, 1990, pp. 81 & 82). |
|
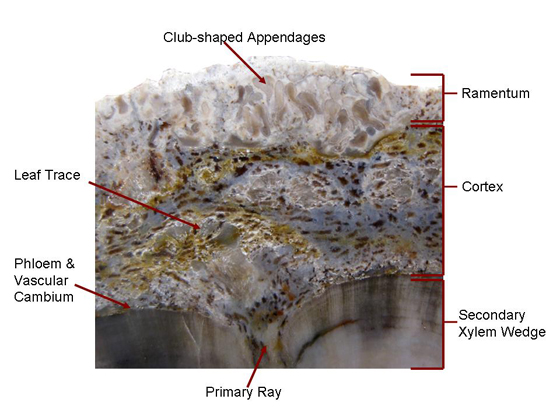 Figure 3 |
|
The woody wedges are formed from secondary xylem, which is made from radially aligned tracheid cells 24-40 µm in diameter. Xylem rays one to two cells wide are spaced between every 4 to 10 rows of tracheids, see Figure 4. Faint growth rings can be seen in some specimens and are thought to be evidence of dry spells or even seasonal changes. The wood boring larvae of reticulated beetles (family Cupedidae) typically live in fungus infested wood. Round grub holes attributed to beetles in this family have been found in the xylem of H. kerkbyorum specimens from East McElmo Creek (Tidwell & Ash, 1990, p. 90). Grub holes in a specimen of H. taylorii measuring 1 mm can be seen in Figure 5. Cupedidae grub holes suggest Hermanophyton grew in a reasonably moist, forested environment (Tidwell & Ash, 1990, p. 88). |
|
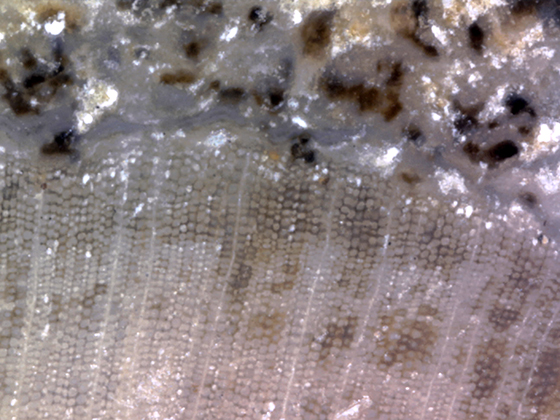 Figure 4 |
|
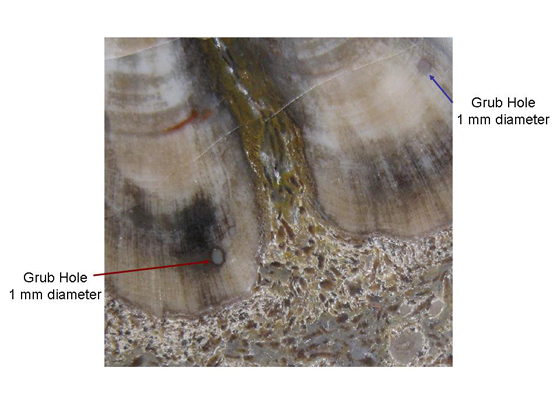 Figure 5 |
|
The structure of the Hermanophyton stem is rather complex. Tangential and radial sections of stems reveal the xylem wedges grow together making connections; however, as the stem length increases the primary rays open back up between the xylem wedges. Leaf traces radiating out from the primary xylem split as they grow outward through the primary ray. Leaf traces also split as they meander through the outer cortex. Fusion of different leaf traces and cortical bundles form the vascular strands of leaf bases at the stems outer surface (Tidwell & Ash, 1990, pp 80-82). The outer surface of well preserved stems is dimpled with leaf bases or scars, see Figure 6. |
|
 Figure 6 |
|
Hermanophyton stems tend to be straight with no branching, see Figure 7. Tidwell and Ash (1990) speculate that Hermanophyton was most likely a small to medium narrow stemmed tree crowned with small leaves which dropped off as the plant grew leaving behind numerous, small, persistent leaf bases (pp. 87 & 88). Evidence suggests that the tree could reach heights 18 meters (Tidwell, 2002, p. 199). Taylor, Taylor and Krings (2009) point to a stem that maintained a 12 cm diameter over a length of 10 m, which might suggests a non-self-supporting growth habit consistent with a large liana (p. 775). Based
upon variations of the generalized anatomy discussed above,
four species
of Hermanophyton have been described and
include H. kirkbyorum, H. taylorii, H.
glismanii, and H. owensii (Tidwell & Ash, 1990, pp. 83-85). The etymology of all
four species names can be traced back to collectors who shared
their collections and field experiences with the scientists
Chester Arnold, William Tidwell and Sidney Ash. |
|
 Figure 7 |
|
Affinities of Hermanophyton No roots, leaves, or reproductive structures of Hermanophyton have been identified, which makes it difficult to determine systematic affinities. Hermanophyton has been compared to Rhexoxylon, a Triassic aged seed fern found in Africa that possesses xylem wedges surrounded by parenchymatous tissue. However, vascular cambium in Rhexoxylon develops on both sides of the wedges; also, anomalous vascular tissue is found in the pith area. These two characteristics clearly separate Rhexoxylon from Hermanophyton. Comparisons have also been made with cycadophytes and present-day lianes (vines). The similarities to these two groups are limited. With the physical evidence at hand we can only say that Hermanophyton is a gymnosperm stem of unknown affinity (Tidwell & Ash, 1990). Geologic Setting Conclusion With
scientific evidence we can imagine the Morrison Basin 150 million years
ago. Herds of herbivorous
dinosaurs roamed the planes in search for food
in the vegetation that surrounded lakes and rivers. The vegetation that surrounded
these areas included bryophytes, cycadeoids, horsetails, ginkgos, conifers,
ferns, and our described Hermanophyton. These ecosystems supported fish,
insects, amphibians, reptiles (such as crocodiles and pterosaurs), and small
mammals. Herds of dinosaurs supported carnivorous dinosaurs, like Allasaurus.
The rock deposits speak of environments subjected to repeated episodes of
drought and flood. Droughts followed by floods formed lake and river deposits
that acted as Nature’s museums, collecting artifacts of extinct life,
providing the curious with a glimpse into ancient ecosystems.
|
|
Bibliography |
|
Daniels, F.J. 1998. Petrified Wood: The World of Fossilized Wood, Cones, Ferns, and Cycads. Western Colorado Publishing Company: USA. Daniels, F.J. and Dayvault, R.D. 2006. Ancient Forests: A Closer Look at Fossil Wood. Western Colorado Publishing Company: Canada. Knoll, H. (2010a). Het Late Krijt van Aken en omgeving: Deel
1-Verkiezeld hout, dennenappels en Meer. Natuurhistorisch
Maandblad 99 (8): 181-185. Taylor, T.N., Taylor E.L. & Krings, M. (2009). Paleobotany: The Biology and Evolution of Fossil Plants [2nd Ed]. New York: Academic Press. Tidwell, W.D. and Ash, S.R. 1990. On the Upper Jurassic Stem Hermanophyton and its Species from Colorado and Utah, USA. Palaeontographica 218, 77-92. Tidwell, W.D. 1998. Common
Fossil Plants of Western North America. [2nd Edition]. Smithsonian Institution Press: Washington,
pgs 214-215. |











