
 |
 |
Permineralized Wood  Schinoxylon sp. Green River Formation Cenozoic; Paleogene; Eocene Blue Forest, Wyoming 12 cm x 9 cm x 1 cm |
|
Permineralized fossils form when solutions rich in minerals permeate porous tissue, such as bone or wood. Minerals precipitate out of solution and fill the pores and empty spaces. Some of the original organic material remains, but is now embedded in a mineral matrix (Schopf, 1975). Bone and wood tissues act as excellent frameworks to preserve cell structure. Silicates, iron oxides, metal sulfides, native elements, carbonates, and sulfates can be involved in permineralization. Permineralization is one of the most faithful modes of fossil preservation. In fact, scientists have tried to replicate the process in the laboratory, but no artificial permineralization is equal to the best natural preservation by cryptocrystalline silica or calcium carbonate (Schopf, 1975). Formation of the finest petrified wood involves permineralization with silica, usually from a volcanic source, along with replacement and recrystallization. During the initial stages of permineralization amorphous silica infills pits connecting cells and pricipitates on cell walls. At this early stage no replacement has occurred. Replacement of cellulose in cell walls may occur as permineralization continues. Cellulose that degrades leaves room for the emplacement of silica between and within cells walls. The more decay resistant lignin that remains in the cell walls continues to act as a guiding framework to preserve structure. Later, silica is deposited in cell lumina, the cavity enclosed by the cell walls, and voids created by wood degradation. Silica
that initially permeates the porous tissue and that which
replaces cell wall material is amorphous. This amorphous
silica is unstable and slowly crystallizes to more stable
forms over millions of years. The transition to more stable
forms of silica involves continued polymerization and water
loss. Higher ordered forms of opal are created through
this process and eventually lead to the thermodynamically
more stable silica quartz (Stein, 1982). The quality
of preservation usually, but not always, declines during
successive stages of silicification (Mustoe, 2003).
In some instances higher ordered opal and chalcedony may
act as the initial replicating minerals (Mustoe, 2008). Petrified wood and petrified dinosaur bone are probably the best known permineralized fossils among the general public. Although not as well known, the coal ball represents a very informative permineralized fossil. A special type of fossil, the coal ball, can be found in the coal deposits of the Pennsylvanian and Permian periods. Coal balls contain swamp vegetation, which has been permineralized with calcium carbonate, preserving 3-D cellular structure. Coal balls are studied in serial section using the cellulose acetate peel method to reveal microscopic structure. Serial sections can be used to reconstruct organs and entire plants. The five major groups of plants found in coal balls include: Lycophytes, sphenopsids, ferns, seed ferns, and cordaiteans (Rothwell, 2002). The in situ preservation of plant materials in coal balls allows paleontologist to study plant associations that tell us something about the palaeoecology of the coal swamps. Coal balls reveal that the arborous fern Psaronius became the dominant canopy tree after the extinction of Lepidondendrales near the Middle Pennsylvanian. Certain species of small ferns and horsetails have been found, which grew in association with the roots of Psaronius (Rothwell, 2002). Bibliography Mustoe, G.E. (2003). Microscopy of Silicified Wood. Microscopy Today, vol 11, no 6, pp. 34-37. Mustoe, G.E. (2008). Mineralogy and geochemistry of late Eocene silicified wood from Florissant Fossil Beds National Monument, Colorado, in Meyer, H.W., and Smith, D.M., [Eds.], Paleontology of the Upper Eocene Florissant Formation, Colorado (pp. 127-140). Geological Society of America Special Paper 435. Rothwell,
G.W. (2002). Coal Balls: Remarkable Evidence of Palaeoxoic
Plants and the Communities in Which They Grew. . In Dernbach,
U. & Tidwell, W.D. Secrets of Petrified Plants: Fascination
from Millions of Years (pp. 39-47). Germany: D’ORO
Publishers. Stein, C.L. (1982). Silica Recrystallization in Petrified Wood. Journal of Sedimentary Petrology, vol 52, no 4. pp. 1277-1282. | |
 Permineralized Limb with Bark Schinoxylon sp. Green River Formation Eocene Blue Forest, Wyoming 12 cm x 10 cm |
 Permineralized Palm Fiber Palmoxylon sp. Bridger Formation Eocene Farson, Wyoming 4 cm diameter |
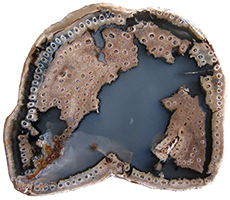 Permineralized Fern Stipe (Petiole) Acrostichum Bridger Formation Eocene Wyoming, USA 3 cm x 2 cm x 2.5 cm long |
 Permineralized Grape Stem Vitaceoxylon Bridger Formation Eocene Wyoming, USA 2 cm x 2 cm x 3.5 cm long |
|
 Permineralized Dinosaur Vertebra Morrison Formation, Jurassic Utah/Colorado 7.5cm x 8cm |
|
|
Introduction
Silicified
Fossil Wood Composition Cellulose, hemicellulose and lignin account for over 95% of the dry weight of wood (Leo & Barghoorn, 1976). The average density of 43 species of softwoods and 96 species of hardwoods examined by Hoadley is 0.53 g/cm3 (Hoadley, 1990). The average density of the softwoods alone was 0.43 g/cm3, while for the hardwoods it was 0.57 g/cm3. Silicified wood generally contains more than 90%, by weight, of silica (Leo & Barghoorn, 1976; Sigleo, 1978; Furuno et al., 1986 II; Mustoe, 2008). Woods mineralized with opal have densities of 2.04 g/cm3 or less. Woods permineralized with quartz have densities of 2.34 g/cm3 or greater (Mustoe, 2008). Leo and Barghoorn (1976) note that many mineralized woods preferentially fracture toward a radially longitudinal plane as do non-mineralized woods. They hypothesized that this is due to retention of wood in the permineralized specimen or to discontinuities in silica deposition predetermined by the orgininal wood structure at the time of petrifaction. How much of the original wood is present? The first well documented attempt to answer this question was carried out by St. John (1927). St. John examined 25 prepared sections of various silicified wood specimens for cell structure under a light microscope. The sections were treated with a solution of one third hydrofluoric acid and two thirds alcohol to remove silica and then reexamined under the microscope. Some specimens retained most or some of the structure indicating the presence of organic matter. Other specimens lost all of their structure with no trace of organic matter. Mustoe (2008 and written personal communication, 2011) employed a more quantitative method to determine the presence of organic matter utilizing heat to destroy residual organic matter and measuring loss in mass. Mustoe concluded that most of the plant tissue is destroyed during silicification. Sigleo (1978) isolated lignin derivatives from 200 Ma Araucarioxylon arizonicum specimens deomonstrating that small traces of relic organic matter can persist after many millions of years. Overall, evidence suggests that very little of the original organic matter remains in silicified wood. How do we account for the visual appearance of cellular detail in silicified specimens that have little to no organic material? Leo and Barghoorn (1976) outline five ways in which cellular detail may be retained in silicified specimens: 1. The actual wood may remain intact after permineralization. Specimens
from the same wood deposit can vary in how much structure is retained.
Even single specimens may exhibit
variation in the retention of wood structure.
The idea that variations in mineralogy lead to differences in texture accords
with my own experience. I have specimens from Sweet Home Oregon that exhibit
beautiful annual rings. Upon closer inspection with a 10x loupe, variations
in cellular detail are immediately apparent. In some areas that have a grainy
texture, described by collectors as “sugary”, no cellular detail
is revealed. In other areas, on the same specimen, the grain size of silica
is so fine that wood histology can be studied with much satisfaction. Do
these differences in how cell structure is preserved represent different
taphonomic pathways or different stages within the same pathway? What are
the conditions and processes that lead to the formation of siliceous petrifactions? The conditions of temperature and pressure during fossil wood formation are equivalent to those found in sedimentary environments of shallow depth. Excessive pressures would deform wood shape and tissues. Excessive temperatures (above 100 degrees Celsius) break down wood substances. The pH of the sediment-laden water within the wood is probably neutral to slightly acidic. Wood chemically breaks down at pH values below 4.5 and above 7. Silica is highly soluble at a pH of above 9 making precipitation less likely. The weathering of volcanic ash may produce a pH that is quite high (alkaline), which would release silica into solution making it available for emplacement in wood as the pH is lowered (Leo & Barghoorn, 1976). These physical and chemical parameters help to define the environmental conditions in which wood can act as a template for silica deposition. A study of silicified wood from the Triassic-aged Chinle Formation of Arizona supports the parameters above. Sigleo (1979) compared the geochemistry of silicified wood and its associated sediments (sandstone with some siltstone and clay) to determine the environmental conditions for the process of wood mineralization. Fossil wood that has stayed in place since deposition and mineralization was carefully chosen for the study. The abundance of minerals and trace elements were measured from the core of the fossil wood to its periphery. Abundance of the same minerals and trace elements were measured in associated sediments and clays from the periphery of the spcimen up to 3 meters away. The clay consisted of 80-90% montmorillonite, which formed from volcanic ash. Identical aluminum concentrations in the clay at the boundary of the fossil and within the periphery of the fossil wood suggest that montmorillonite co-precipitated with silica. The abundance of most trace elements increased from the core of the fossil to its periphery with some notable exceptions. Antimony (Sb) was found to be much more abundant within the fossil wood than in surrounding sediments with its highest concentrations at the core where carbon was also the most abundant. The abundance of uranium was highest in the carbonaceous core and decreased to the periphery of the fossil wood. Uranium and antimony are more mobile when in an oxidized state, but precipitate when reduced. In contrast, the abundance of manganese, which is more soluble in a reduced state, was low within the fossil specimen and had its highest concentration with in the sediments adhering to the branch. Clays in the associated sediments were depleted of rare earth elements (REE) relative to the total sediments. REE can be leached from clays in soils with a pH of 4-5. Sigleo (1979) concluded that the geochemistry of the fossil wood and associated sediments supports a fossil-forming environment that was anoxic and slightly acidic. The silicification process occurred at surface temperatures and pressures associated with typical surface and groundwater conditions. There was no evidence of alkaline conditions or exceptionally high silica concentrations in solution. A continuous supply of silica in solution was provided by the hydrolysis of volcanic ash. Later we will see evidence for secondary stages of silicification associated with hydrothermal events in Chemnitz fossil wood. Leo and Barghoorn (1976) developed a hypothetical model for wood silicification based upon wood anatomy, a low temperature laboratory process for incipient silicification, and inferred geochemical parameters found in natural settings. Leo and Barghoorn hypothesized that there is a chemical affinity between wood and silica through hydrogen bonding. Several observations support the idea that relative unaltered wood may actually serve as a silica sink through hydrogen bonding. Coalified wood does not silicify well as it has lost functional groups capable of hydrogen bonding. Carbonate, sulfide, and fluoride petrifactions are less common and of lower quality possibly because these ions do not establish hydrogen bonds as well as silica. Silicified woods are often encased within a matrix that is not cemented with silica, suggesting a differential affinity for silica between the wood and the surrounding sediment. Leo and Barghoorn’s experiments with artificial silicification of wood, described later, also support the idea that wood has an affinity for silica. According to their hypothesis, when wood is permeated by silica solution, hydrogen bonding links silicic acid to hydroxyl groups on cellulose making up the inner cell walls. As water is lost silicic acid is polymerized into opal. Layers of silica are deposited with the wood acting as a template (pp. 22-25). Initially, silica is fixed to the inner cell walls and along the perimeter of the tracheid lumen and infill pits connecting adjacent tracheids. During initial stages of permineralization silica deposition fills voids, especially the lumina. Cell walls may be replaced with silica in later stages of silicification. To replicate cell structure with high fidelity a balance between wood degradation and mineral deposition must be achieved. The amorphous silica that initially permeates wood is highly hygroscopic (attracts water) and highly permeable to fluid flow (Leo & Barghoorn, 1976). The rate at which this silica crystallizes to more impervious forms is extremely slow in geologic terms. Thus, long after silicification begins the influx of silica and the migration of degraded organic matter can continue through this porous medium. As silicification proceeds to more advanced stages cellulose degrades leaving more room for the emplacement of silica between cells and within cell wall layers. Lignin, under anaerobic conditions, is the most decay resistant compound in wood and continues to act as a template for structural detail. In fact, fossil woods show an increase in the ratio of lignin to holocellulose (cellulose & hemicellulose) when compared with contemporary counterparts. Specimens aged Eocene or older are devoid of holocellulose. Thus, lignin is the last organic matter to be replaced. The balance between removing organic matter and the deposition of minerals is often not complete so, some organic matter remains in many petrifactions. As the process continues silica deposits in intercellular spaces and voids created by shrinkage and wood degradation. Silica that initially fixes to the wood structure is amorphous. This amorphous silica is unstable and slowly crystallizes to more stable forms. The transition to more stable forms of silica involves continued polymerization and water loss. Higher ordered forms of opal are created through this process and eventually lead to the thermodynamically more stable silica quartz (Stein, 1982). Multiple studies have examined natural wood petrifactions representing different fossil deposits providing insights into the physical and chemical processes involved in wood silicification. Many of these studies lend evidence in support of Leo and Barghoorn's model of silicification. Buurman (1972) examined fossil wood specimens preserved with a variety of minerals using X-ray diffraction, optical and scanning electron microscopy. We will summarize his findings relating to silicification. In one group of silicified woods Buurman found evidence suggesting that wood preservation is best when disordered tridymite (opal-CT) replaces cell walls or when this opal is subsequently transformed to chalcedony through recrystallization. In both instances, Buurman suggests the fossil wood has formed by replacement rather than filling. A second group of silicified woods preserved with chalcedony and quartz retained some woody tissue. Buurman suggested that these specimens had formed through permineralization (filling). Buurman concluded that replacement and permineralization are distinct processes. In
a more detailed study, Scurfield and Segnit (1984) examined 75 fossil wood specimens from Australia
using X-ray diffraction, differential thermal analysis,
electron probe techniques, optical and scanning electron
microscopy. Their study found that replacement of the
cell walls of tracheids and vessels occurred in addition
to permineralization. They conclude that petrifaction
of wood occurs in five stages, summarized as follows: Furuno et al. (1986 I & II) used light microscopy, SEM, electron probe X-ray microanalysis (EPMA), polarizing microscopy, and X-ray diffraction to study the anatomy and mineralization of Pliocene and Miocene silicified woods collected in Japan. Chemical and physical parameters such as color, reaction to hydrofluoric acid on fractured surfaces, and loss on ignition were also examined. Three samples representing the Pliocene were collected from the Mukaiyama Foramation. Three samples representing the Miocene were collected from the Hata, Fujina, and Kori Formations. Two Sequoia petrifactions of Pliocene age are black in color. When fractured radially and treated with hydrofluoric acid these specimens produced silica casts of tracheid lumens. The cell walls of these specimens are not silicified and retain substantial organic matter. Cell lumens and pit chambers are permineralized with opal-CT and some quartz, with one specimen showing more quartz than the other. By weight one specimen is 22.89% organic matter and 77.11% silica, while the other is 20.56% organic matter and 79.44% silica. A third Tsuga petrifaction of Pliocene age is white to light brown in color. When fractured radially and treated with hydrofluoric acid isolated silicified tracheids were produced. The cell walls of this specimen are preserved in silica. The lumens and cell walls are preserved in opal-CT and some quartz. By weight the specimen is 2.42% organic matter and 97.68% silica. One Podocarpus petrifaction of Miocene age is black in color. Cell lumens and cell walls are preserved in mostly quartz. The Podocarpus specimen contains some carbon in the cell walls and in resin cells. The authors hypothesize that resin within the resin cells may have fossilized into amber. By weight the Podocarpus specimen is 5.93% organic matter and 94.08% silica. A second Miocene aged Tsuga specimen is light grey in color. The Miocene-aged Tsuga has cell lumens and walls preserved in quartz. By weight this specimen is 0.09% organic matter and 99.91% silica. One Celtis petrifaction of Miocene age is the only angiosperm dicot in the study and is light grey to brown in color. The Celtis specimen has cell lumens and cell walls preserved in quartz. By weight the Celtis specimen is 0.90% organic matter and 99.10% silica. Hydrofluoric acid applied to radially fractured surfaces on Miocene samples did not produce lithomorphs as easily as the Pliocene samples. The samples in this study show a correlation between specimen color and carbon content. The study also shows a correlation between the extent of silicification and time. Specimens in this study exhibit a pattern of increasing quartz content over time. Furuno et al. (1986 I & II) proposed the following phases for the silica mineralization of wood. 1. Deposition of silica filling voids like cell lumens and pit chambers, but no penetration of silica into the walls, which remain. The authors note that phase 2 was not observed in the specimens of their study. Furuno et al. (1986 II) present the following senario for wood silicification. First opal-A dissolved in an alkaline solution that permeated the wood. Silica precipitated within the acidic wood. Opal-CT was formed. The mineral was kept in a solid state and over tens of millions of years the opal-CT was crystallized to quartz. In the course of time all of the silica minerals were transformed to quartz. Jefferson (1987) studied the preservation of Cretaceous-aged, silicified conifer wood from Alexander Island, Antarctica. Microscopic examination was made of acetate peels and thin sections made in the transverse, tangential longitudinal, and radial longitudinal planes. Fractured surfaces, which broke preferentially in the radial plane, were examined using SEM. EDAX was utilized to identify the amount of silica and organic materials present in the cell walls as well as the distribution of minerals filling cell lumina. Alexander wood in which silica infiltrated lumina without penetrating cell walls is poorly preserved. Cell walls in these specimens are reduced to thin lines of carbon inclusions embedded in silica. Well-preserved Alexander wood resulted from a process in which silica infiltrated cell lumina and impregnated cell walls. Cell walls in well-preserved specimens contain between 82 and 87% silica with only scattered carbonized residue remaining. Jefferson surveys how the preservation and form of bordered pits along fractured longitudinal surfaces can be used to determine the extent to which silica infiltrated pit chambers. For example, silica infilling creates casts of pit chambers. Silicified microfibrills making up the cell walls of Alexander wood are 20 to 150 times larger in diameter than extant conifers. Tracheids of Alexander wood often contain preserved fungi hyphae and lensoid-ovoid organic bodies interpreted as fungal spores. The author suggests that cell walls of Alexander wood were delignified by fungal activity seperating microfibrils, making them available for silica coating. Growth of silica on fibrillar bundles exposed by fungal activity allowed cell wall structures to be preserved. Thus, cell walls were not replaced; rather, they were preserved through a process of silica infiltration and impregnation enhanced by biogenic degradation. Euhedral, cubic iron sulfied crystals enclosed in silica are found within the lumina of some tracheids. The growth of pyrite crystals indicates an initial reducing environment. Quartz crystals within cell lumina most often grow inward without penetrating cell walls. In some lumina apatite and collophane fill up the remaining space not occupied by quartz. Thus, it appears that the cell walls were already silicified when cell lumina were mineralized. Furthermore, the presence of apatite and collophane probably represent a major change in the composition of the permineralizing solution. Jefferson proposed a two-phase silicification process for Alexander petrifactions. 1. Silica impregnated cell walls. Biogenic delignification and decay of the cell wall opened up spaces and isolated microfibrils creating interfibrillar porosity. Silicic acid precipitated and polymerized onto the microfibrils and filled the interfibrillar porosity. The amorphous silica coating microfibrils crystallized into chalcedony and microcrystalline quartz. The author references work that states the subsequent transformation and ordering of silica would be expected to take millions of years. Jefferson concluded that Alexander wood supports the two-stage theory of wood silicification proposed by Leo and Barghoorn (1976). However, results from the study of Alexander wood suggest that the impregnation of cell walls can be promoted by biogenic degradation. Some of the specimens were permineralized with only
opal-CT, others were a combination of opal-CT and chalcedony,
and still others were quartz. In specimens permineralized
with both opal-CT and chalcedony the two silica phases
appeared to coexist as primary minerals. Thus, evidence
for the transformation of opal-CT to chalcedony was
missing. Evidence gathered by this study suggests that
the Florissant specimens were directly mineralized
with chalcedony. The precipitation of opal, chalcedony,
and quartz are influenced by concentrations of dissolved
silica. Opal is precipitated with high concentrations
of dissolved silica while chalcedony is precipitated
with low concentrations and quartz still lower. Mustoe
speculates that these geochemical characteristics may
explain the patterns of mineralization found at Florissant. Mustoe
concluded that petrification at Florissant occurred
in several
stages. Spaces between adjacent tracheids were often unmineralized, making the fossil wood permeable to water and susceptible to cleaving radially, tangentially and transversely from freeze-thaw weathering. This finding has important implications to the preservation of specimens at Florissant Fossil Beds National Monument. Mustoe’s findings are important because they suggest that petrifaction may occur through multiple processes or pathways. Witke et al. (2001) used Raman and cathodoluminescence spectroscopy to study the chemical composition and structural order of silicified plants from the 290 million year old Chemnitz Petrified Forest. Samples of the seed fern Medullosa, gymnosperm Dadoxylon, sphenopsid Calamodendron striatum, and tree fern Psaronius were used in the study. Tracheid and sclerenchyma cells making up vascular tissues are preferentially preserved in Chemnitz fossil plants. Cell walls are preserved primarily in microcystalline alpha quartz. In areas where tissue is not preserved as as in the pith of Medullosa one finds banded agate structures composed of phanerocystalline and microcrystalline alpha quartz along with moganite. A very low percentage of original organic material remains in the Chemnitz silicified plants. Raman spectra of carbonized material dispersed within Chemnitz petrifcations reveals a rank equal to bituminous and anthracite coals. Witke et al. (2001) proposed the following steps for the mineralizations of Chemnitz petrifcations. Dietrich et al. (2013) used backscatter electron imaging (BSE) and electron backscatter diffraction (EBSD) in a scanning electron microscope to study the ordering of silica preserving xylem tissue in three different fossil tree forms found in Chemnitz Petrified Forest. The study of Chemnitz petrifactions included the root mantle of the tree fern Psaronius, vascular segments in the pith of the seed fern Medullosa stellata, and the secondary xylem of the gymnosperm Dadoxylon. Former open spaces and specific tissue structures were found to be correlated with different silica ordering. Tracheid cell walls inthe aerial roots of Psaronius are largely preserved by well-ordered microcrystalline quartz grains. The thickened cell walls of the sclerenchyma fibers, making up the outer boundary of the aerial root, are preserved with extremely fine-grained but well-ordered quartz. Cell lumina are filled with cryptogrystalline quartz. Tracheid cell walls in M. stellata exhibited multiple microcrystalline layers outlining the former cell wall layers. In fact, crystal growth directions within these layers seem to mirror former cellulose fibril orientations. As in Psaonius, cell lumina were largely filled with cryptocrystalline quartz. Tracheid cell walls in the secondary xylem of Dadoxylon are preserved with microcrystalline quartz. Cell lumina are filled mostly with cryptocrystalline quartz or more infrequently with microcrystalline quartz or large fluorspar crystals. In general the cell lumina of all three specimens are filled with cryptocrystalline quartz, while the cell walls were preserved with microcrystalline quartz. Microcrystalline quartz grain size decreased with tracheid cell diameter. Psaronius tracheid cell walls are preserved with the largest grain size, while the cell walls of Psaronius sclerenchyma, M. stellata tracheids, and Dadoxylon tracheids are preserved with a finer grain size. The tissues of these plants vary in their proportions of cellulose, hemicellulose, and lignin. The results of this study suggest that tissue composition affects silicification ordering in petrifactions. Dietrich et al. (2013) proposed the following stages of petrifaction to explain the patterns of silica ordering observed in the tissues of Chemnitz specimens. 1. Silica solution permeates the porous xylem tissue and forms a silica gel inside the tracheid lumina starting at the inner cell walls. Is there a place we can go to examine the silicification of wood occurring in more recent times? Karowe and Jefferson (1987) investigated the initial stages of silicification by examining trees buried by Mount St. Helens lahars or mudflows dated at 1980, 1885, A.D. 1450-1550, and 36,000 years B.P. Wood samples were examined using scanning electron microscopy and energy dispersive X-ray analyses. Wood buried in 1980 showed no significant mineral deposition. Wood buried in 1880 and A.D. 1450-1550 exhibited traces of silica on cell walls as well as cell wall decay. Wood buried 36,000 years B.P. showed silica impregnation of cell walls. Decay in these older specimens affecgted the secondary wall and removed the middle lamella. Karowe and Jefferson concluded that the increase in silica deposition as well as decay associated with the age of these trees supported the model of silicification proposed by Leo and Barghoorn in 1976. The apprent increase in silica deposition was an exciting find. However, researchers at the University of Bonn were unable to reproduce the results of Karowe and Jefferson even when using specimens prepared from the same trees (Hellawell et al., 2011). Perhaps Karowe and Jefferson were looking at an instrumental artifact. Scientists at the University of Bonn are currently working on a paper that will explore the results of their study in more depth. It is clear that many petrified wood deposits, such as those found at Yellowstone, are associated with volcanic mudflows. George Mustoe has examined many Holocene wood specimens from mudflows and has found no evidence of silicification (Mustoe, written personal communication, 2012). It is equally clear that wood can be quickly mineralized under the right conditions. Timbers in copper mines from Cyprus and Arizona have been found that contain copper (Daniels & Dayvault, 2006). It would be interesting to study the amount of original wood, extent of permineralization and lithification in these specimens and compare them with silicified woods. Wood buried in ash produced from the 1886 eruption of Mt. Tarawera in New Zealand is mineralized with silica. Wood specimens recently exposed to hot springs in Yellowstone exhibit the beginning stages of silicification; however, geologic evidence suggests siliceous thermal springs have not been major sites of wood petrifaction (Leo & Barghoorn, 1976). Silicified wood from Wyoming contained enough organic material to be carbon dated at less than 3,000 B.P. X-ray diffraction reveals that these recently silicified woods are impregnated with amorphous opal (Leo & Barghoorn, 1976; Stein, 1982). The question of how long it takes to form petrified wood also depends on the physical qualities of the fossil wood we have in mind. Are we looking at wood that is mostly intact and has cell lumina and intercellular spaces impregnated with minerals or fossil wood that has little to no wood remaining and has cellular detail replicated in opal-CT, chalcedony, and microgranular quartz? If we have in mind the latter then these recent petrifactions are not what collectors think of as gem quality petrified wood, which can be cut and polished at the lapidary. The
initial emplacement of silica as a film may occur rapidly.
Artificial silicification of wood in the lab and studies
of natural silicified wood demonstrate that the physical
state of silica in newly formed petrifactions is amorphous
(Leo & Barghoorn, 1976). Conversion of this
silica to increasingly stable forms of opal-A (amorphous),
to opal-CT (cristobaite & tridymite), to chalcedony
(cryptocrystalline quartz), and finally to microgranular
quartz requires millions of years (Leo & Barghoorn,
1976; Stein, 1982; Kuczumow et al.,
1999). Under normal conditions conversion of
opal to quartz requires tens of millions of years; however,
under geothermal conditions the same process may occur
in 50,000 years or less (Mustoe, 2003). Fascination with natural permineralized specimens has sprurred interest in creating methods for artificial petrifaction. Artificial petrifaction reflects both scientific and commercial interests. Methods for artificial petrifaction have been devised as ways to study wood structure and model the initial stages of natural silicification. Artificial petrifaction is also used to develop wood composites and ceramics. Leo and Barghoorn (1976) document early attempts at creating artificial silicified wood in the 1500's by Basil Valentine and Johannes Kentmann.Experimental replication of these early recipes has not been successful. More recent attempts have produced some positive results. Ryan W. Drum from the University of Massachusetts, Amherst, describes his attempt at the laboraty silicification of twigs in a 1968 article that appeared in the journal Science. Twigs were soaked in solutions of sodium metasilicate, washed, and then treated with chromic acid to remove organic remains. Entire twigs did not remain intact; however, single cells and small aggregates of cells were replicated in silica. Drum described these replicas as very fragile and included electron micrographs of his results. Drum goes on to say that his in vitro silicification process might provide a method to study cellular spaces in 3-D, intercellular connections, and the morphology of woody cells. Leo and Barghoorn (1976) improved upon Drum's experiments. Their procedure included boiling wood to degas and waterlog specimens. The wood was alternately soaked in solutions of water and ethyl silicate at neutral pH and 70 degrees Celsius. Ethyl silicate decomposes to monomolecular silicic acid, which is thought to be the silicifying agent in natural processes. Nitric acid and potassium chlorate were used to remove organic matter. The silica lithomorphs that remained replicated cell structure and were more substantial than those produced from Drum's procedure. The silica lithomorphs were fragile and composed of amorphous silica. These silica lithomorphs resemble the first stages of permineralization observed in recent silicified specimens. Persson et al. (2004) evaluated the use of sol-gel mineralization to study the wood morphology of spruce and birtch xylem. Wood chips of birch, Betula verrucosa, and spruce, Picea abies, along with the pulp of spruce were used in the study. Spruce pulp was prepared using the Kraft process. The Kraft process uses a mixture of sodium hydroxide and sodium sulfide along with cooking at high tempertures (170 degrees Celsius) to degrade lignin and hemicellulose, converting the wood to a pulp consisting of almost pure cellulose fibers. Wood chip samples and the pulp were soaked in ethanol and vacuum dried two times to facilitate impregnation. The prepared wood and pulp samples were immersed in a sol-gel mixture (polysilicic acid) for three days at 60 degrees Celsius. Silica casts of the wood and pulp were recovered by removing all organic material by heating to 575 degrees Celsius for 6 hours. The brittle silica casts replicas were studied using environmental scanning electron microscopy (ESEM) and transmission electron microscopy (TEM). Persson et al. (2004) found that their sol-gel casting medium penetrated and condensed on the ultrastructure of cell walls. The high-resolution silica casts were used to observe the three dimensional structure of xylem and pit structures connecting wood cells. Experiments in artificial silicification described above have been used as methods to replicate the process of natural incipient silicification and to study the ultrastructure of wood. These experiments suggest that the organic cell structure of wood acts as a template for initial silica deposition. The fact that wood is removed in making silica-cast replicas limits the use of these procedures for studying advanced stages of natural silicification. More recently, Ballhaus et al. (2012) designed closed-system experiments at 100 degrees Celsius to simulate the silicification of trees buried by volcanic pyroclastics. Silica rich solutions were prepared by reacting powdered obsidian with water at 100 degrees Celsius for several days. Under these conditions it was found that silica and alkali oxides readily go into solution while aluminum oxide remains in the residue. The pH of the solution increases to values of 9.4-10.5. Wooden cubes made of Douglas-fir (Pseudotsuga menziesii) were reacted with the silica solution in an autoclave for up to 300 hours. In the presence of the wood the pH and silica concentrations decreased, while alkalis remained in solution. Silica rich solutions were again prepared by reacting powdered obsidian with water at 100 degrees Celsius for several days. The solution was doped with NaOH to increase the pH values to 12.5 and 13.2 in order to increase the solutions silica concentration. Douglas-fir wood cubes were reacted with the solutions in autoclaves for 112 days. Periodically, slices of wood were prepared and analyzed for precipitates. After several days many of the cell lumina were infilled with silica. Most precipitates were found on wood that exhibited final pH values near neutral. Under SEM the silica precipitates appeared as microspheres of opal. These experiments simulate and provide evidence in support of processes thought to be involved in the incipient permineralization of wood. Volcanic material rich in siliceous glass can be quite alkaline. When these solutions come into contact with wood the pH is lowered and silica becomes less soluble, precipitating onto wood surfaces. This pH gradient between the silica rich alkaline water and the acidic environment of the wood tissue acts as force to precipitate opal onto wood surfaces. A second force driving permineralization is the ability of wood tissue to extract silica out of solution. The decrease in the silica concentration in the presence of Douglas-fir wood cubes is indirect evidence that is consistent with Leo and Barghoorn’s (1976) hypothesis that silica species chemically bond with hydroxyl groups on wood surfaces. Ballhaus
et al., (2012) used simple diffusion and advection models
to estimate
how long it would take to permineralize
a tree with opal. Using Frick’s Law a theoretical conifer
tree trunk with a diameter of 100 cm and a length of 100 cm
buried horizontally in a pyroclastic deposit would be permineralized
through diffusion within an estimated time of 47,000 years.
Using an advection model the same tree buried upright (in
situ)
would require approximately 3,600 years for cell lumina and
intercellular spaces to become impregnated with opal. This
estimate assumes wood structure remains intact. The results
of this study are consistent with other findings and indicate
that the incipient permineralization of large trees with opal
is on a time scale of thousands of years. Yongsoon Shin and colleagues at the Pacific Northwest National Laboratory (PNNL) developed a method for creating a silica-based ceramic that mimics wood structure. The process uses surfactants and silicate solutions to mineralize wood that has been soaked in an acid solution. After the silicate solution penetrates cell wall structures it is heated to high temperatures in air to oxidize the silicate and remove organic residue. This method creates a ceramic, which faithfully reproduces cellular structures in great detail as confirmed by SEM images (Shin et al. 2001). Shin et al (2001) points out that, “Another important phenomenon related to the current study is natural petrification, which takes place over a very long period of time. In some cases the cellular tissue is completely replaced by silicate and other minerals. Our study not only points to a more rapid approach to transforming organic tissues into ceramic materials, it may also shed some light on how natural petrification takes place.” An article on ChemicalProcessing.com entitled ‘Petrified wood yields super ceramics’ describes a process developed at PNNL for using wood to form the ceramics silicon carbide (SiC) and titanium carbide (TiC). The process involves soaking the wood in acid, infusing it with titanium or silicon, and baking it in an argon-filled furnace at 1,400 degrees Celsius. This process is the same as Shin's 2001 study except for heating in an argon atmosphere instead of air. The 2001 and 2005 experiments used small blocks of pine and poplar wood. Both the macro and microstructure of the wood is preserved in this ceramic. The material has the strength of steel, and can resist temperatures of up to 1,400 degrees Celsius. PNNL scientist Yongsoon Shin is quoted as saying that one-gram of this material flattened has enough porosity to cover an entire football field. SEM and TEM images were used to study the microscopic structure of these ceramics (Shin et al. 2005). The SiC ceramics could be used for making filters, catalysts, cutting tools, abrasives, and coatings. The purpose of Shin’s research is to develop methods for using natural biological materials as templates to construct inorganic materials. Hamilton Hicks was issued US patent 4612050 on September 16, 1986 for a mineralized sodium silicate solution used to create wood with the “non-burning characteristics of petrified wood” (Patent Storm). In one experiment a horse stall was set on fire with combustible materials. The treated wood showed signs of charring, but did not burn. The patent indicates that the treated wood is non-toxic and has an inherently bad taste. This bad taste prevents horses from “chewing or nibbling the wood to shreds”. The inventor speculates, “petrifaction of the treated wood is achieved when minerals in his solution “replace the cells” and the solution hardens the wood. It would be interesting to compare the amount of wood still present as well as the nature and extent of silicification in this product with that found in naturally silicified wood. Products referred to as "instant petrified wood" may provide insights in the initial stages of permineralization. However, many of the materials and procedures used to make these products are not found in nature. Furthermore, procedures to make silica-cast replicas and ceramics remove wood after the initial permeation with artificial mediums. Products made from the initial emplacement of silica, represented by both artificial and recent natural petrifactions do not resemble what a collector regards as gem quality petrified wood. Multiple lines of evidence suggest that natural fossil wood silicified with opal-CT, chalcedony, and microgranular quartz requires millions of years to form. ConclusionEvidence for pathways that lead to the formation of silicified wood comes from studies of fossil wood representing different aged deposits, laboratory procedures for artificial petrifaction, and examination of trees buried recently by volcanic deposits. The formation of silicified wood includes permineralization, replacement, and recrystallization. A summary of an accepted model for natural silicification is as follows. When wood is permeated by silica solution, hydrogen bonding links silicic acid to cellulose making up the inner cell walls. Silicic acid is polymerized into opal through water loss. Silica that initially fixes to wood structure is amorphous and can recrystallize to a more stable form. Layers of silica are deposited with the wood acting as a template. Initially, silica is fixed to the inner cell walls and infills the lumina of tracheary elements (vessel elements and or tracheids) and the pits connecting adjacent tracheary elements. Cell walls may be replaced with silica as permineralization continues. To replicate cell structure with high fidelity a balance between the chemical and biological decomposition of wood and the precipitation of silica must be achieved. As silicificaiton proceeds to more advanced stages cellulose degrades leaving more room for the emplacement of silica between cells and within cell wall layers. This replacement is not molecule-by-molecule. Lignin is the most decay resistant compound in wood and continues to act as a template for structural detail. Later, cracks and fractures are preserved with silica. A traditional model of silicification includes a transformation of the initial silica to increasingly more stable forms from opal-A to opal-CT to chalcedony and finally to quartz. However, evidence for silica recrystallization is lacking in some specimens suggesting more than one pathway for silicified wood formation (Mustoe, 2008). Studies of silicified wood from Florissant and Chemnitz demonstrate that cell walls and lumina can be preserved in different forms of silica. Thus, forms of silica that serve as the initial replicating material for cell walls and open spaces may be different from what initially permineralized the wood. Factors that affect what form of silica serves as the initial replicating material will lead to a better understanding of natural silicification pathways. One of these factors may include the plant tissues themselves. Examination of microstructure in petrifactions of Chemnitz encourages further investigations into how tissue composition affects what form of silica may act as a replicating material (Dietrich et al. 2013). Acknowledgements I would like to thank George Mustoe, Dagmar Dietrich, Yongsoon Shin, and Jim Mills for their expertise and encouragement. Post Script Our readers might find the following recent articles helpful in exploring the silicification of wood. Petrifactions and Wood-Templated Ceramics: Comparisons Between Natural and Artificial Silicification Late Tertiary Petrified Wood from Nevada USA: Evidence of Multiple Silicification Pathways Opalized Wood from Clover Creek, Gooding County, Idaho Multi-Stage Silicification of Pliocene Wood: Re-Examination of an 1895 Discovery from Idaho, USA. Mineralogy of Paleocene Petrified Wood from Cherokee Ranch Fossil Forest, Central Colorado, USA. The Bruneau Woodpile: A Miocene Phosphatized Fossil Wood Locality in Southwestern Idaho, USA Wood Petrifaction: A New View of Permineralization and Replacement Mineralogy of Non-Silicified Wood A Silicified Carboniferous Lycopsid Forest in the Colorado Rocky Mountains, USA The Blue Forest of Ancient Lake Gosiute: Sweetwater County, Wyoming |
|
|
|
Ballhaus, C., Gee, C.T., Bockrath, C., Greef, K., Mansfeldt, T, and Rhede D. (2012). The silicification of trees in volcanic ash-An experimental study. Geochimica et Cosmochimica Acta 84: 62-74. Buurman,
P. (1972). Mineralization of Fossil Wood. Scripta Geologica,
vol. 12, pp. 1-43. Daniels, F.J. and Dayvault, R.D. (2006). Ancient Forests: A Closer Look at Fossil Wood. Western Colorado Publishing Company: Canada. Dernbach, U. (1996). Petrified Forests: The World’s 31 Most Beautiful Petrified Forests. D’ORO: Germany. Dernbach, U. & Tidwell, W.D. (2002). Secrets of Petrified Plants: Fascination from Millions of Years. D’ORO: Germany. Dietrich, D., Lampke, T., & Rossler, R. (2013). A microstructure study of silicified wood from the Permian Petrified Forest of Chemnitz. Palaontologische Zitschrift: Scientific Contributions to Palaeontology. Berlin Heidelberg: Springer-Verlag. DOI 10.1007/s12542-012-1=0162-0. Furuno T., Watanabe T., Suzuki N., Goto, T., Yokoyama, K. (1986). Microstructure and Silica Mineralization in the Formation of Silicified Woods I. Species identification of silicified woods and observations with a scanning electron microscope. Journal of the Japan Wood Research Society. Vol 32, No 6, pp 387-400. Japan: Mokuzai Gakkaishi. Hellawell,
J., Gee, C. T., Ballhaus, C., Clynne, M. A. and Sander,
P. M. (2011). Silicification of wood: identifying ancient
and present-day processes. Abstract for poster presentation,
The Palaeontological Association 55th Annual Meeting, Plymouth
University, UK. Jefferson, T.H. (1987). The Preservation of Conifer Wood: Examples from the Lower Cretaceous of Antarctica. Palaeontology, vol 30, part 2, pp. 233-249. Kerp,
H. (2002). The Rhynie Chert: The Oldest and Most Completely
Preserved Terrestrial Ecosystem. In Dernbach, U. & Tidwell,
W.D. Secrets of Petrified Plants: Fascination from
Millions of Years (pp. 23-27). Germany: D’ORO
Publishers. Mustoe, G.E. (2001). Washington’s Fossil Forests. Washington Geology, vol 29, no1/2, pp. 10-20. Mustoe, G.E. (2003). Microscopy of Silicified Wood. Microscopy Today, vol 11, no 6, pp. 34-37. Mustoe, G.E. (2008). Mineralogy and geochemistry of late Eocene silicified wood from Florissant Fossil Beds National Monument, Colorado, in Meyer, H.W., and Smith, D.M., [Eds.], Paleontology of the Upper Eocene Florissant Formation, Colorado (pp. 127-140). Geological Society of America Special Paper 435. Mustoe, Written Communication, March 2011. Mustoe, G.E. Written Communication, November 2012. Patent
Storm. US Patent 4612050 - Sodium silicate composition, Ranson, J.E. (1955). Petrified Forest Trails: Guide to the Petrified Forests of America. Oregon: Mineralogist Publishing Company. Persson, P.V., Fogden, A., Hafren, J., Daniel, G. & Iversen, T. (2004). Silica-Cast Replicas for Morphology Studies on Spruce and Birch Xylem. IAWA Joural. Vol 25, pp. 155-164. Scurfield, G. & Segnit, E.R. (1984). Petrification of Wood by Silica Minerals. Sedimentay Geology, 39, 149-167. Shin, Y., Liu J., Chang J.H., Nie Z., & Exarhos, G.J. (2001). Hierarchically Ordered Ceramics Through Surfactant-Templated Sol-Gel Mineralization of Biological Cellular Structures. Advanced Materials, 13, pp. 728-731. Shin,
Y., Wang, C., & Exarhos, G.J. (2005). Synthesis of
SiC Ceramics by the Carbothermal Reduction of Mineralized
Wood with Silica. Advanced Materials, 17, pp.
73-76. Sigleo, A.C. (1979). Geochemistry of Silicified Wood and Associated Sediments, Petrified Forest National Park, Arizona. Chemical Geology, 26, pp. 151-163. St. John, R.N. (1927). Replacement vs. Impregnation in Petrified Wood. Economic Geology, vol 22, pp. 729-739. Stein, C.L. (1982). Silica Recrystallization in Petrified Wood. Journal of Sedimentary Petrology, vol 52, no 4. pp. 1277-1282. Witke, K., Gotze, J., Rossler, R. Dietrich, D. Marx, G. (2004). Raman cathodoluminescence spectroscopic investigations on Permian fossil wood from Chemnitz--a contribution to the study of the permineralization process. Spectrochimica Acta Part A, 60, pp. 2903-2912.
|
|











